I am looking for: Learn arrow down
I am looking for:
-
- Agriculture, Food & Natural Resources
- Architecture & Construction
- Arts, Audio/Video Technology & Communications
- Business Management & Administration
- Education & Training
- Finance
- Government & Public Administration
- Health Science
- Hospitality & Tourism
- Human Services
- Information Technology
- Law, Public Safety, Corrections & Security
- Manufacturing
- Marketing
- Science, Technology, Engineering & Mathematics
- Transportation, Distribution & Logistics
- View All
Categories
Categories
Search For
Moles
Conversion Between Mass and Moles of an Element (Screencast)
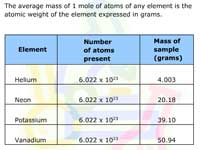
Atomic weights are used to convert the mass of a sample into the number of moles of the element in the sample and vice versa. Four examples are provided for practice.
By Dr. Miriam Douglass Dr. Martin McClinton
The Mole and Avogadro's Number
Learners examine how chemists use moles to "count" atoms by weight. Examples are given.
By Debbie McClinton Dr. Miriam Douglass Dr. Martin McClinton
Gas Volume and Molar Amount
In this brief object, learners examine the direct relationship between the volume of a gas sample and the number of moles of gas. A problem is presented so students can test their knowledge of Avogadro's Law.