I am looking for: Learn arrow down
I am looking for:
-
- Agriculture, Food & Natural Resources
- Architecture & Construction
- Arts, Audio/Video Technology & Communications
- Business Management & Administration
- Education & Training
- Finance
- Government & Public Administration
- Health Science
- Hospitality & Tourism
- Human Services
- Information Technology
- Law, Public Safety, Corrections & Security
- Manufacturing
- Marketing
- Science, Technology, Engineering & Mathematics
- Transportation, Distribution & Logistics
- View All
Categories
Categories
Search For
Taffy Atoms
Students read brief descriptions of atoms, molecules, elements, and compounds, and complete a matching exercise that pictures these particles and molecules as pieces of taffy.
Oxidation Numbers
Learners assign oxidation numbers to atoms in neutral compounds and in polyatomic ions. Six examples are worked through in detail, and three problems are provided.
By Debbie McClinton Dr. Miriam Douglass Dr. Martin McClinton
Ions
Ions are electrically charged particles obtained from an atom or from a chemically bonded group of atoms by adding or removing electrons. Eight examples illustrate the number of protons, neutrons, and electrons in positive ions (cations) and in negative ions (anions).
By Debbie McClinton Dr. Miriam Douglass Dr. Martin McClinton
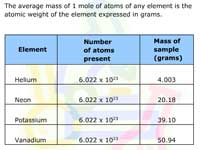
The Mole and Avogadro's Number
Learners examine how chemists use moles to "count" atoms by weight. Examples are given.
By Debbie McClinton Dr. Miriam Douglass Dr. Martin McClinton